Research Highlights
On January 22, a research team led by Associate Professor Yin Qin from the Faculty of Pharmaceutical Sciences at Shenzhen University of Advanced Technology (and concurrently a researcher at Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences) developed anovel strategy for the asymmetric transfer hydrogenation of quinolines based on dynamic kinetic resolution (DKR). This strategy efficiently constructs structurally novel chiral tetrahydroquinolines, significantly expands the chemical space of N-heterocycles, provides a new material foundation for drug discovery, and serves as a paradigm for DKR-based transformations of other aromatic heterocycles. The results have been published online in the Journal of the American Chemical Society.
Screenshot of the published article
Research assistant Liang Mingrong and jointly supervised PhD student Du Xian are co-first authors. Yin Qin is the sole corresponding author, with the Faculty of Pharmacy, Shenzhen University of Advanced Technology as the corresponding affiliation.
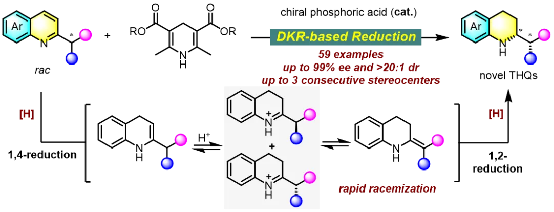
Schematic of the research achievement
Drug discovery relies heavily on lead compounds. Identifying biologically active lead compounds through screening is the foundation of innovative drug research. yet efficiently building diverse novel compound libraries remains a major bottleneck in drug development. Statistics show that over 90% of FDA-approved small-molecule drugs (2015–2020) contain nitrogen heterocycles, with about one-third featuring at least one chiral N-heterocycle—making chiral N-heterocycles true “privileged scaffolds” in small-molecule therapeutics.
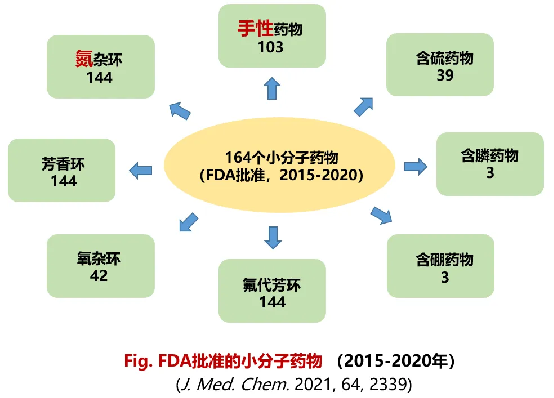
Distribution of small-molecule drugs
Chiral tetrahydroquinolines, a key class of N-heterocycles, are widely found in natural products and pharmaceuticals, drawing significant interest in their asymmetric synthesis. Current methods primarily target structures with single or multiple in-ring chiral centers, whereas efficient synthesis of medicinally promising tetrahydroquinolines bearing adjacent in-ring and exocyclic chiral centers remains rare.
To tackle the challenges of constructing multi-chiral N-heterocycles and controlling stereoselectivity, Yin Qin’s team proposed a novel“dynamic kinetic resolution-based asymmetric transformation of heteroarenes” strategy. In previous work, the team achieved efficient construction of indolines bearing adjacent exocyclic and endocyclic chiral centers via asymmetric hydrogenation of electron-rich indoles (J. Am. Chem. Soc. 2024, 146, 5081–5087.) Building on this, the team targeted the ubiquitous tetrahydroisoquinoline motif and developed chiral phosphoric acid-catalyzed DK R-based asymmetric transfer hydrogenation of quinolines, enabling efficient synthesis of diamines or amino alcohols bearing adjacent exocyclic and endocyclic chiral centers. The products can be further transformed into diverse N-heterocycles.
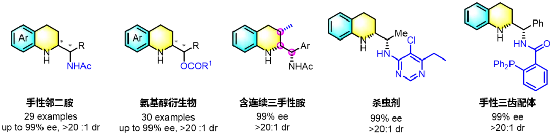
Representative product molecules
According to Yin Qin,
this study features three major highlights:
↓↓↓
1. The method provides an efficient route to novel chiral tetrahydroquinolines bearing adjacent in-ring and exocyclic chiral centers, delivering products in excellent yield, diastereoselectivity (dr), and enantioselectivity (ee) (59 examples, up to 99% ee and >20:1 dr). It even enables construction of triply contiguous chiral centers, dramatically expanding tetrahydroquinoline chemical space and offering promising new scaffolds for drug discovery.
2. Mechanistic studies reveal that DK R relies on rapid acid-promoted interconversion among in situ-generated endocyclic/enamine, exocyclic enamine, and imine intermediates. A plausible hydrogen-bonding transition-state model accounts for the excellent diastereoselectivity, with DFT calculations confirming that catalyst–substrate hydrogen bonding governs both enantio- and diastereocontrol.
3. The reaction is scalable to gram quantities, and products are readily transformed into potential insecticides, chiral tridentate ligands, and other tetrahydroquinolines bearing contiguous chiral centers.
·

Group website

Paper link