Hydrogen is widely regarded as a clean and efficient energy carrier. If we could easily produce large amounts of hydrogen, it would revolutionize both our lives and the environment!
Recently, the team led by Academician Cheng Huiming (Academician Workstation, Chinese Academy of Sciences) and Assistant Professor Peng Jing (School of Materials Science and Energy Engineering, Shenzhen University of Advanced Technology) published groundbreaking results inAngewandte Chemie International Edition, revealing the critical role of Anti-site Defects in FeWN2 Nanosheets for Enol Electrooxidation Coupled with H2 Evolution.

In today’s energy research, developing efficient and sustainable hydrogen production methods is a top priority. Biomass electrooxidation-coupled hydrogen evolution stands out because it lowers the anodic overpotential while co-producing valuable chemicals. However, cleaving the strong C–H and O–H bonds in biomass is difficult, causing current systems to struggle at industrial-level current densities and hindering scalability.
To tackle this challenge, the Cheng Huiming and Peng Jing teams developed a ternary layered FeWN₂ electrocatalyst rich in Anti-site Defects (ASDs), dramatically boosting ascorbic acid (AA) oxidation performance. Experimental results show that in a two-electrode electrolyzer at 60 °C, a current density of2.5A/ cm⁻² is achieved at just 0.69 V (vs. RHE). When the voltage is increased to 1.12 V, the current density reaches an impressive 4 A/cm⁻² with 100% Faradaic efficiency for hydrogen evolution — meaning virtually every electron participating in the reaction is used to produce hydrogen, with zero waste!
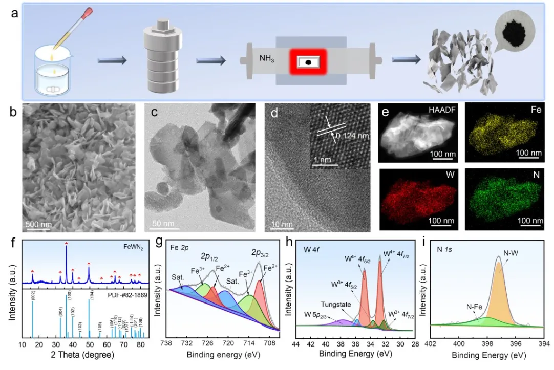
Figure 1 | Preparation and microstructure of FeWN₂. a Schematic illustration of the synthesis of nitrided nanosheets. b SEM, c TEM, and d HRTEM images.e HAADF-STEM image and corresponding elemental mapping of FeWN₂. f XRD pattern of FeWN₂ with the standard powder diffraction file. g–i XPS spectra of Fe 2p, W 4f, and N 1s.
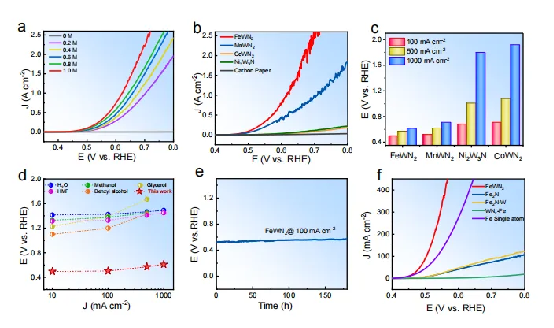
Figure 2 | Electrochemical performance a LSV polarization curves at different AA concentrations. b LSV polarization curves of AAOR using different MWN₂ catalysts in 1 M AA solution at a scan rate of 5 mV s⁻¹. c Performance comparison of MWN₂ at 10, 100, and 500 mA cm⁻². d Performance comparison of various noble-metal-free electrocatalysts at 10, 100, 500, and 1000 mA cm⁻². e Long-term stability of FeWN₂ at 100 mA cm⁻² for 180 hours in 1 M AA electrolyte. f LSV polarization curves of Fe-based and W-based catalysts in 1 M AA solution at a scan rate of 5 mV s⁻¹.
Using atomic-resolution characterization techniques, the team discovered abundant Anti-site Defects (ASDs) in the FeWN₂ nanosheets — like tiny built-in “switches” inside the material. Compared with similar catalysts, FeWN₂ far outperforms in ascorbic acid (AA) oxidation, maintaining exceptional stability for 180 hours at 100 mA cm⁻².
Why is FeWN₂ so powerful?
The answer came from theoretical calculations.
Anti-site Defects (ASDs) between Fe and W create uneven charge distribution, making it easier for ascorbic acid (AA) to adsorb on the surface and be rapidly activated into dehydroascorbic acid (DHA). Meanwhile, W atoms near the Fe sites replenish electron density, maintaining optimal Fe–DHA adsorption strength and dramatically boosting catalytic efficiency.
Thisstudy provides fresh ideas for developing high-performance organic oxidation catalysts, highlights the enormous practical potential of FeWN₂ nanosheets, and is expected to drive electrocatalytic hydrogen production toward industrial application, strongly supporting sustainable energy development.
Chen Zhengjie and Shao Qiting are co-first authors; Cheng Huiming and Peng Jing are co-corresponding authors.