Rheumatoid arthritis (RA) is a common, highly prevalent autoimmune inflammatory disease in which the immune system attacks healthy joint tissue, characterized by synovitis. Overexpression of inflammatory cytokines triggers massive inflammatory cell infiltration, leading to synovial inflammation, pannus formation, cartilage and bone erosion, and irreversible joint damage. Disability rates in moderate-to-severe cases reach 60–70%. Even more alarming, advanced RA affects extra-articular organs (interstitial lung disease, rheumatoid nodules, nervous and cardiovascular systems), posing a severe threat to patients’ physical and mental health.
However, the unclear pathogenesis of RA makes early diagnosis and treatment extremely challenging.


On April 15, the team led by Professor Zhang Peng (Faculty of Biomedical Engineering, Shenzhen University of Advanced Technology), together with researchers Liu Chengbo and Chen Jingqin from Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences, published inCell Reports Medicine, developing a new technology for targeted diagnosis and treatment of inflammatory cells and achievingdynamic tracing and precise regulation of synovial macrophages in RA. (click “Read more” at the end of the article for paper details).

Screenshot of the published article

This study is the first to reveal the critical role of the RhoA gene in pro-inflammatory and osteoclastogenic functions of synovial macrophages, providing innovative tools for early personalized diagnosis and treatment of RA and other major inflammatory/immune diseases, as well as a brand-new direction for precise disease prevention and control.
In synovial tissues from RA patients and model mice, RhoA protein was found to be significantly upregulated in macrophages — a finding that may open new therapeutic avenues for RA-associated bone damage.
Using single-cell RNA sequencing, immunofluorescence, and RA mouse models, the team showed that RhoA is rapidly activated in bone-marrow-derived macrophages under RANKL/M-CSF stimulation and directly participates in osteoclastogenesis.
These results highlight the pivotal role of RhoA in RA synovial macrophages, suggesting it as a promising therapeutic target and providing crucial insights into RA immunopathology and novel treatment strategies.

To investigate the effect of RhoA on osteoclast differentiation and joint destruction, the team generated macrophage-specific conditional knockout mice (RhoA-cKO) using the Cre-LoxP system. The mice developed normally and bred according to Mendelian ratios. Successful knockout was confirmed by DNA electrophoresis. In collagen-induced arthritis models, RhoA-cKO mice exhibited markedly reduced joint swelling, lower paw thickness, and lower clinical scores — comparable to non-immunized controls — indicating that macrophage-specific RhoA deletion prevents inflammation and edema. Immunohistochemistry and Western blot confirmed significantly reduced RhoA expression in bone-marrow-derived macrophages, validating knockout efficiency.
The study demonstrates that macrophage-specific deletion of RhoA effectively prevents joint destruction in collagen-induced arthritis (CIA) models, establishing RhoA as a promising therapeutic target for reducing bone damage in rheumatoid arthritis (RA).

Moreover, the research team developed an advanced gene-editing system named SMART-Cas9 that specifically edits the RhoA gene in joint macrophages of rheumatoid arthritis. Through non-invasive live-animal fluorescence imaging, the system was proven to precisely target and accumulate in arthritic joint macrophages in living rats.
This study confirms RhoA as a key regulator of osteoclast formation and a promising therapeutic target for rheumatoid arthritis. The team developed an innovative strategy namedSMART, which uses macrophage membrane vesicles rich in phosphatidylserine (PS) to safely and precisely deliver CRISPR-Cas9 into macrophages, achieving efficient RhoA gene editing with minimal toxicity.
SMART-Cas9 significantly reduces osteoclast-mediated joint damage by targeted editing of RhoA in macrophages in inflammatory arthritis. Thus,SMART not only offers new insights into RA treatment but also paves a pathway for gene therapy development.
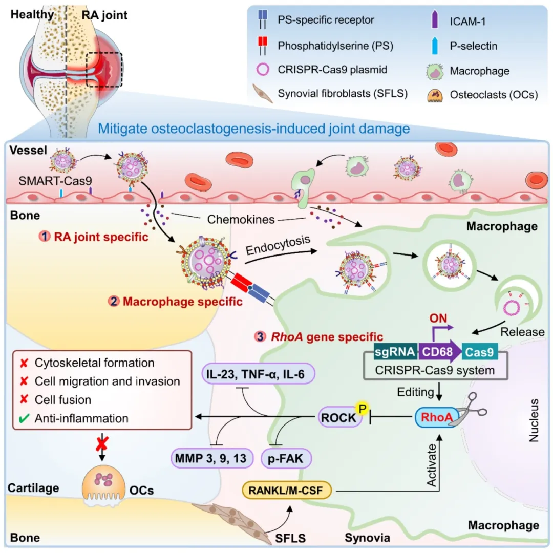
Schematic illustration of the study
Zhang Peng, Chen Jingqin, and Liu Chengbo are corresponding authors; Chen Jianhai (Guangdong Medical University) and Tan Jianwei (Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences) are co-first authors.
The study was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the State Key Laboratory of Medical Imaging Science and Technology, CAS Youth Innovation Promotion Association, Guangdong Basic and Applied Research Foundation, and Shenzhen Science and Technology Program.