Metal halide perovskites(MHPs), thanks to their unique optoelectronic properties, have emerged as leading materials for next-generation solar cells, LEDs, X-ray and γ-ray detectors, and other devices. Compared to polycrystalline thin films, single-crystal perovskites have fewer defects and exhibit significantly superior performance.
However, how these high-quality crystals grow step-by-step in solution and what exactly happens at the crystal-solution interface have long been challenging questions for scientists.
Recently,
an answer has been found.


The team led by Assistant Professor Shi Zhifang from the Faculty of Materials Science and Energy Engineering at Shenzhen University of Advanced Technology has made a major breakthrough in the microscopic mechanisms of crystal growth. Their latest findings have been published inNature Synthesis(click “Read more” at the end of the article for paper details). The study reveals a fascinating phenomenon during solution-based perovskite crystal growth: a self-regulated “cold protective layer” at the crystal facet, providing a new approachfor preparing high-quality perovskite single crystals and developing future high-performance materials.

Screenshot of the published article

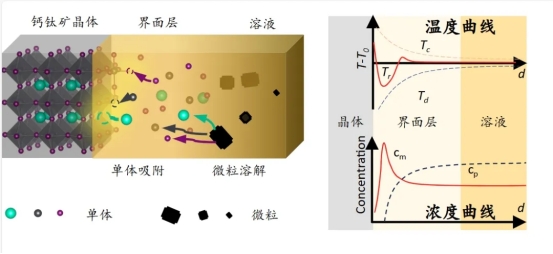
Using in-situ fluorescence lifetime imaging microscopy (FLIM) and micro-absorption spectroscopy, combined with high-precision microscopy and temperature measurement, the teamsuccessfully mapped the temperature distribution near the growth interface of MAPbI₃ perovskite crystals at the micrometer scale.
“Surprisingly, we discovered a ‘cold zone’ 1.5–4 μm from the crystal edge where the temperature was significantly lower than the bulk solution—this is the ‘cold protective layer’ at the crystal edge,” said Shi Zhifang. According to Shi Zhifang, this cold protective layer acts like an “invisible barrier” that prevents disordered particles in the solution from adhering to the crystal surface, shielding the surface from external interference and ensuring smooth, uniform crystal growth.
How does this cold protective layer form? During crystal growth, heat is released (exothermic crystallization), but surrounding particles act like “heat-absorbing sponges,” absorbing this heat through dissolution (endothermic dissolution). Meanwhile, crystal growth continuously consumes monomers nearby, lowering their concentration and driving further particle dissolution, creating a continuous endothermic cycle. Ultimately, this low-temperature region forms a barrier that allows the crystal to grow “calmly” in solution, keeping disordered particles at bay, preventing impurity interference, and reducing defect formation.
“It’s like setting up a cordon around a construction site,” explained Professor Shi Zhifang. “Only qualified materials are allowed into the construction zone, ensuring the crystal grows orderly in the correct direction.”
Mechanism of perovskite crystal growth
To validate this mechanism, the researchers conducted a“stirring experiment.”When a magnetic stirrer disrupted the growth environment, the cold protective layer was destroyed, resulting in disordered morphology and unstable facets. The experiment confirmed the importance of minimizing disturbances during growth for achieving high-quality crystals and further validated the critical role of the cold protective layer.
“This work not only experimentally reveals the microscopic regulation mechanism of perovskite crystal growth in solution but also provides a theoretical foundation and technical roadmap for the controllable growth of high-quality crystals in the future,”said Shi Zhifang. Moreover, the theory may be extended to other particle-containing solution systems, potentially driving technological advances in related fields.