Structural diversity is a key driver of drug innovation. Nitrogen-containing heterocycles are the most prevalent privileged scaffolds in small-molecule drugs; developing novel N-heterocyclic scaffolds is crucial for discovering new drug molecules.
On May 15, Associate Professor Yin Qin’s team (Faculty of Pharmacy, Shenzhen University of Advanced Technology & Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences) reported inJournal of the American ChemicalSociety a “sequential asymmetric transfer hydrogenation” strategy to construct novel continuous-chiral tetrahydroquinoline and tetrahydro quinoxaline scaffolds. This breakthrough significantly expands the chemical space of nitrogen heterocycles and holds great promise for new drug discovery, especially for 3D-structured drug molecules.
This marks the second JACS publication from Yin Qin’s team in a short period, following their recent report on“Dynamic Kinetic Resolution-Based Asymmetric Hydrogenation of N-Heteroarenes” (J. Am. Chem. Soc. 2025, 147, 4239−4248.; J. Am. Chem. Soc. 2024, 146, 5081–5087.)
PhD candidate Zhang Mangang (jointly trained by Shenzhen University of Advanced Technology and Central South University) is the first author; Yin Qin is the sole corresponding author, with Shenzhen University of Advanced Technology as the corresponding affiliation. Dr. Niu Tianyu and others made key contributions.
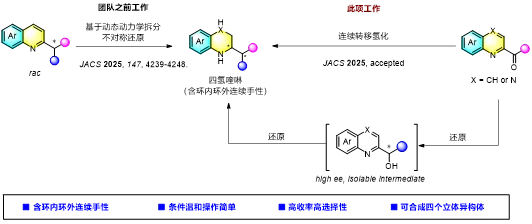
Figure 1. Overview of the research
Structural diversity has long been a core focus of drug discovery. Identifying biologically active lead compounds through screening is the foundation of innovative drug research. To accelerate lead discovery, the “escape from flatland” strategy has been proposed to expand 3D chemical space and reduce reliance on planar molecules. Traditional planar molecules are easy to synthesize but often suffer from poor selectivity and off-target effects. In contrast, 3D chiral molecules act as precise “keys” that better fit biological targets, improving efficacy and reducing toxicity.
Among chiral synthesis methods, asymmetric hydrogenation (AH) is a highly efficient and reliable tool for converting planar molecules into 3D scaffolds. Over the past two decades, direct asymmetric hydrogenation of readily available aromatic N-heterocycles (NHCs) has advanced remarkably, enabling rapid access to C(sp3)-rich saturated N-heterocycles. Quinoline, with its relatively low aromaticity, has attracted particular attention and is readily reduced by various chiral catalysts. The resulting chiral 1,2,3,4-tetrahydroquinolines (THQs) are core scaffolds in numerous natural products and anticancer/antibacterial drugs.
However, current asymmetric hydrogenation of quinolines is limited to simple alkyl/aryl substituents and can only construct in-ring chiral centers, severely limiting product diversity — like producing parts with a single mold, far from meeting the structural complexity needed in drug discovery.
To address this, Yin’s team previously developed a dynamic kinetic resolution-based asymmetric transfer hydrogenation of racemic 2-substituted quinolines, yielding novel THQs with adjacent in-ring and exocyclic chiral centers. However, it required pre-installation of a chiral substituent and could only access one pair of diastereomers.
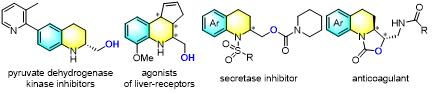
Figure 2. Bioactive molecules containing the chiral tetrahydroquinoline (THQ) scaffold
Building on this foundation and their long-term interest in expanding N-heterocyclic chemical space, the team set a new goal: achieve stereo divergent synthesis of all four stereoisomers of tetrahydroquinolines from simpler substrates. They developed a “C2-acyl quinoline sequential asymmetric hydrogenation” strategy that sequentially reduces the carbonyl and quinoline ring, enabling efficient synthesis of novel THQs bearing both in-ring and exocyclic adjacent chiral centers.
Three major challenges were overcome:
(1) developing a catalyst that efficiently reduces both carbonyl and quinoline under identical conditions;
(2) suppressing catalyst poisoning by bidentate amino-alcohol products;
(3) achieving precise enantio- and diastereocontrol.
After systematic exploration, an iridium-catalyzed sequential asymmetric transfer hydrogenation system was established, delivering 32 examples with excellent enantiocontrol (>90% ee, >20/1 dr). The method was extended to quinoxalines, yielding 10 continuous-chiral tetrahydroquinoxalines.
Mechanistic studies confirmed a cascade pathway of “carbonyl first, then quinoline skeleton,” with stereochemistry controlled by the catalyst at each step. Crucially, controlling temperature and reductant amount allows controllable synthesis of chiral alcohol intermediates, and switching catalyst configuration enables access toall four stereoisomers of the tetrahydroquinoline products.
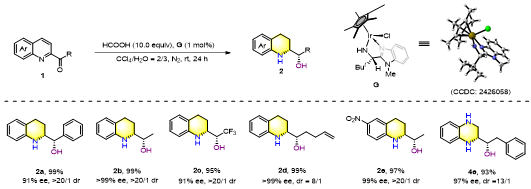
Figure 3. Representative examples
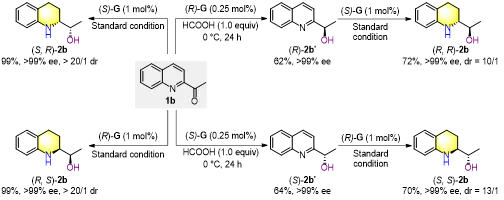
Figure 4. Stereo divergent synthesis of all four enantiomers
According to Yin Qin, thisstudy establishes a highly efficient sequential asymmetric transfer hydrogenation of C2-acyl quinolines and quinoxalines, delivering a series of novel chiral 1,2,3,4-tetrahydroquinolines and tetrahydroquinoxalines with adjacent in-ring and exocyclic chiral centers in excellent yield, enantioselectivity, and diastereoselectivity — dramatically expanding nitrogen heterocyclic chemical space. The core breakthrough of this technology lies in the use of a water-soluble chiral amino-benzimidazole iridium catalyst that enables the sequential reduction of carbonyl groups and heteroaromatic rings. Mechanistic studies show that both reduction steps are precisely controlled by the catalyst without involving dynamic kinetic resolution. This method enables the controllable synthesis of all four stereoisomers of chiral tetrahydroquinolines. The team is currently extending this sequential reduction strategy to other heteroaromatic ring systems, aiming to provide a new paradigm for expanding the chemical space of chiral heterocyclic compounds.
Paper link:
https://pubs.acs.org/doi/full/10.1021/jacs.5c04856
Introduction to Yin Qin’s research group:
https://www.x-mol.com/groups/yin_qin