Recently, the team led by Associate Professor Yin Qin from the Faculty of Pharmacy at Shenzhen University of Advanced Technology and researcher at the Institute of Pharmacology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, reported for the first time a palladium-catalyzed asymmetric coupling reaction initiated by difluorocarbene transfer and developed a new strategy based on palladium-catalyzed difluorocarbene transfer. This method efficiently constructs a library of novel chiral spiro-indolone compounds, which exhibit certain anticancer activity (unpublished).
The study highlights the advantages and potential of difluorocarbene in synthesis and provides a mechanistically unique route for asymmetric carbonyl cyclization reactions. The results have been published online in the Nature journal Nature Communications.
Paper link: https://doi.org/10.1038/s41467-024-52392-5
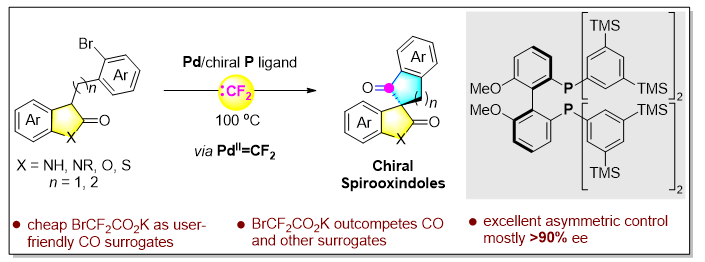
Figure 1. Schematic of the research
Chiral spiro-indolones are a highly important class of organic heterocyclic compounds found in numerous natural product molecules (Figure 2).
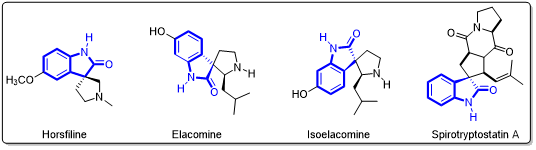
Figure 2. Natural product molecules containing chiral spiro-indolone scaffolds
Moreover, owing to their unique molecular architecture, chiral spiro-indolones are widely used in organic synthesis and medicinal chemistry. Drug molecules containing chiral spiro-indolone scaffolds have been extensively applied in research on anti-inflammatory, antiviral, antimicrobial, and anticancer agents (Figure 3). Therefore, developing efficient synthetic methods for chiral spiro-indolones is of great significance.
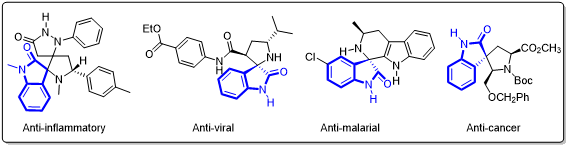
Figure 3. Drug molecules containing chiral spiro-indolone scaffolds
Difluorocarbene can be generated from inexpensive and readily available precursors under mild conditions and is a highly versatile and reactive intermediate in organic synthesis. Typically, it is mainly used to prepare gem-difluoromethyl-containing molecules (Figure 4).

Figure 4. Difluorocarbene precursors and their conventional transformations
Recently, using simple difluorocarbene precursors, transition-metal-catalyzed formation of metal-difluorocarbene (M=CF₂) species and their controlled conversion into valuable chemical products have seen initial progress. For example, the group of Zhang Xingang at Shanghai Institute of Organic Chemistry reported the first palladium-difluorocarbene-involved catalytic coupling reaction and isolated and characterized the palladium-difluorocarbene (Pd(0)=CF₂) species. By fine-tuning reaction conditions, the controllable synthesis of four types of fluorinated compounds was achieved, including difluoromethyl, tetrafluoroethyl aromatic compounds, aryl difluoromethyl, and aryl tetrafluoroethyl ketones. Interestingly, in the presence of water, difluorocarbene can serve as a CO surrogate in carbonylation reactions (Figure 5).

Figure 5. Work by Zhang Xingang’s group
Building on this, the group of Song Qiuling at Fuzhou University reported palladium-catalyzed selective carbonylation coupling of aryl halides and alkynes, in which difluorocarbene acted as a CO donor to produce a series of ynone and γ-butenolide compounds (Figure 6).
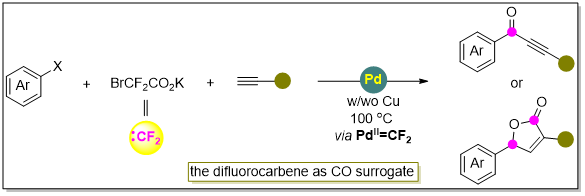
Figure 6. Work by Song Qiuling’s group
Despite these advances, controlling reactivity and stereoselectivity in metal-catalyzed difluorocarbene transfer reactions remains highly challenging: (1) Difluorocarbene is a highly reactive electrophilic species and may be trapped by nucleophiles in the system before coordinating with the metal, leading to undesired byproducts; (2) Metal-difluorocarbene (M=CF₂) species are also highly reactive and prone to various side reactions; (3) Releasing difluorocarbene from inexpensive precursors such as BrCF₂CO₂K/Na typically requires heating, making enantioselective control of the difluorocarbene transfer process extremely difficult. Literature survey indicates that asymmetric coupling reactions initiated by difluorocarbene transfer remain unexplored.
Inspired by the ability of Pd(II)=CF₂ intermediates to generate carbonyl groups in situ with water, the Yin Qin team reports for the first time a palladium-catalyzed asymmetric coupling reaction initiated by difluorocarbene transfer, affording a series of structurally novel chiral spiro-indolones with enantiomeric excesses generally exceeding 90%. In this reaction, the difluorocarbene precursor serves as a practical, easy-to-handle, safe, and highly efficient CO surrogate. More importantly, experiments revealed that BrCF₂CO₂K outperforms gaseous CO and several common CO surrogates in this transformation. The proposed mechanism involves stepwise in situ release of difluorocarbene, rapid migratory insertion of the ArPd(II)=CF₂ species, followed by defluorinative hydrolysis to introduce the carbonyl group. Compared with conventional CO-based carbonylation reactions, this transformation exhibits distinct mechanistic features and potential advantages. Other highlights include:
(1) The method provides an efficient and practical route to chiral spiro-indolones with excellent yields and ee values, tolerating a wide range of aryl substituents (>25 examples, up to 99% ee). The generation of these novel scaffolds expands chemical space and facilitates new drug discovery (Figure 7).
(2) Preliminary mechanistic studies ruled out the formation of free CO during the reaction, confirming that BrCF₂CO₂K is superior to gaseous CO and several common CO surrogates. This work clearly demonstrates the advantages and potential of difluorocarbene in synthesis and offers a mechanistically unique pathway for asymmetric carbonylative cyclization reactions.
(3) The reaction is amenable to gram-scale synthesis, and the products can be readily transformed into other derivatives useful in organic synthesis and medicinal chemistry.

Figure 7. Substrate scope
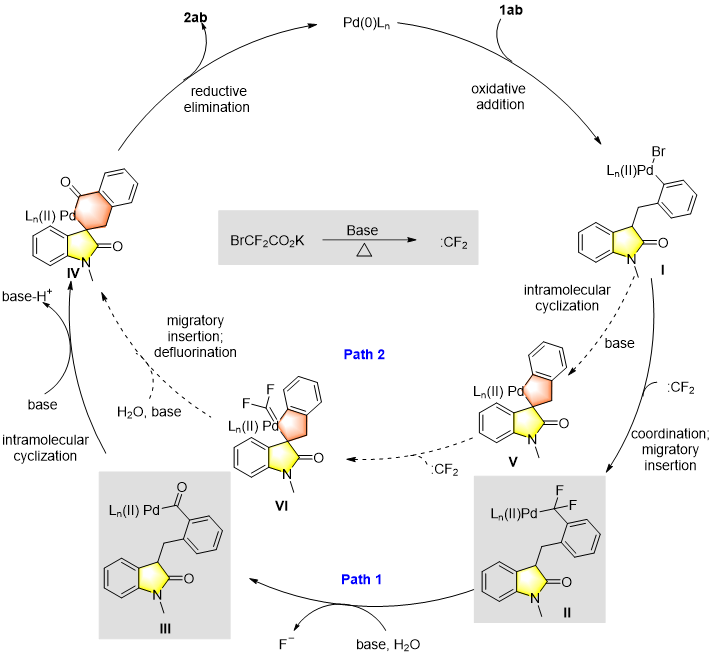
Figure 8. Proposed reaction mechanism
The team creatively integrated difluorocarbene transfer with asymmetric carbonylation, enabling efficient asymmetric synthesis of chiral spiro-indolones. This approach rapidly constructs libraries of structurally novel compounds, significantly expanding the chemical space of chiral spiro-indolones and providing new molecular scaffolds for drug development. It also offers a mechanistically unique avenue for exploring other asymmetric carbonylation reactions.
Assistant Professor Nie Zhiwen from Shenzhen University of Advanced Technology, jointly supervised student Wu Keqin, and research assistant Zhan Xiaohang are co-first authors. Professor Yin Qin is the sole corresponding author, and Shenzhen University of Advanced Technology is the first corresponding institution. Other co-authors also made important contributions to this work.
Recruitment:
The Yin Qin group is recruiting 1–2 postdoctoral researchers. Requirements: Candidates should have a background in organic or medicinal chemistry, with priority given to those with strong track records in asymmetric catalysis, organometallic chemistry, or medicinal chemistry.
Long-term recruitment of jointly supervised graduate students and interns (no quota limit). Requirements: current graduate students in chemistry with strong interest in organic synthesis and medicinal chemistry; diligent, responsible, self-disciplined, and committed to further academic development.
Group website: https://www.x-mol.com/groups/yin_qin