The hypothesis that Parkinson’s disease pathology originates in the gut was first proposed by German neuroanatomist Heiko Braak in 2003.
On September 5, the team led by Professor Ye Keqiang from the Faculty of Life and Health Sciences at Shenzhen University of Advanced Technology, in collaboration with Professor Wu Shengxi and Researcher Xiang Jie from Air Force Medical University, published new findings in Neuron. By establishing a novel gut-specific Parkinson’s disease mouse model, they provided strong evidence supporting this hypothesis.
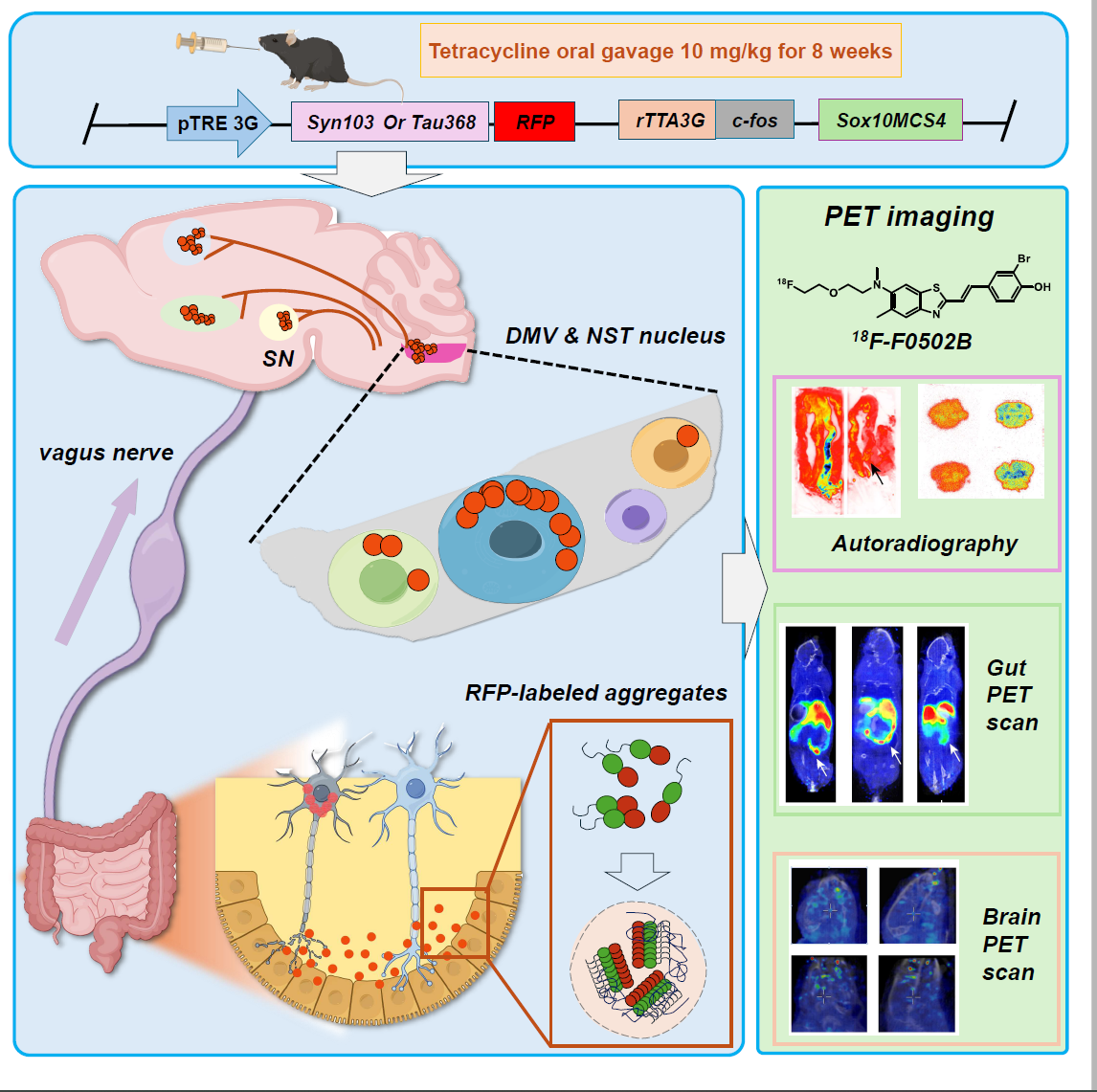
Figure 1: Research schematic. Conditional induction of SNCA/MAPT transgene expression in enteric neurons allows observation of pathological aggregate formation in gut-origin neurons and their propagation to the brain via the vagus nerve. Gut aggregates can also be preliminarily detected using the[18F]-F0502B tracer.
Parkinson’s disease is a common neurodegenerative disorder characterized by motor dysfunction, primarily attributed to dopaminergic neuron loss and extensive α-synuclein aggregation. Studies have detected α-synuclein aggregates in the gut of patients long before they appear in the brain. In recent years, exogenous injection of α-synuclein into the gut has partially validated the hypothesis, but robust animal models have been lacking, and the formation and propagation mechanisms of gut-derived aggregates remain unclear.
Ye Keqiang’s team developed a novel transgenic mouse model with inducible α-synuclein expression in enteric neurons. They found that gut-derived α-synuclein spontaneously aggregates and propagates via the vagus nerve to brainstem regions including the dorsal motor nucleus of the vagus and nucleus tractus solitarius, affecting catecholaminergic and cholinergic neurons, and eventually spreading to broader brain areas. The mice exhibited time-dependent dopaminergic neuron loss, gastrointestinal dysfunction, motor impairments, and cognitive deficits.
Xiang Jie explained that, compared with conventional fibril-injection models, this new “gut-to-brain axis” neurodegenerative disease model offers three key advantages: (1) Conditional induction of pathogenic proteins avoids interference from high endogenous Tau/α-synuclein background; (2) Fluorescent tagging distinguishes propagated from locally induced proteins; (3) Specific induction in enteric neurons enables detailed investigation of gut-derived pathogenic protein effects on brain nuclei.
“Interestingly, Tau protein alone exhibits a similar propagation pattern, suggesting that Alzheimer’s disease may follow a comparable gut-to-brain spreading mechanism,” said Ye Keqiang.
The study also compared propagation speed, extent, and behavioral changes between dual-transgene and single-transgene mice, confirming that co-expression forms aggregate complexes that spread faster and more extensively, providing critical evidence for synucleinopathies and highlighting the complexity and diversity of protein aggregates in pathogenesis.
Notably, the team applied their newly developed α-synuclein aggregate probe[18F]-F0502B to detect gut aggregates in mice with promising results (Cell, 2023), expanding the probe’s potential applications and offering a new tool for early diagnosis of Parkinson’s disease.
“This study allows us to investigate the pathogenesis and neural circuits of gut-origin neurodegenerative diseases and provides a new theoretical foundation for early diagnosis,” said Xiang Jie.
Shenzhen University of Advanced Technology is the first corresponding institution. Xiang Jie, Tang Jingrong, and Kang Fei from Air Force Medical University are co-first authors. Ye Keqiang, Wu Shengxi, and Xiang Jie are corresponding authors. The work received strong support from Professor Wang Jing at Air Force Medical University.