Rheumatoid arthritis (RA) is the most common chronic inflammatory arthritis, characterized primarily by synovitis and destruction of bone and cartilage. Although treatments have advanced significantly, some patients remain unresponsive or only partially responsive to conventional therapies, and long-term use often leads to side effects and drug resistance. Thus, developing new therapeutics remains essential. Controlling inflammation, reducing tissue damage, and promoting bone repair are of great clinical importance. Overexpression of angiopoietin-like protein 4 (ANGPTL4) is considered a driver of RA progression. Initially identified as a lipid metabolism regulator, ANGPTL4 is cleaved in vivo into N-terminal (nANGPTL4) and C-terminal (cANGPTL4) fragments. The cANGPTL4 fragment is involved in lipid-independent processes, including angiogenesis and inflammation.
On October 3, 2024, the team led by Professor Zhang Peng (Shenzhen University of Advanced Technology), in collaboration with Assoc. Professor Cheng Wenxiang (Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences) and Dr. Li Liang (Southern University of Science and Technology), published a paper titled “Bone-protective effects of neutralizing angiopoietin-like protein 4 monoclonal antibody in rheumatoid arthritis” inMolecular Therapy (IF = 12.1).
The study first observed significantly elevated ANGPTL4 levels in serum and synovial tissues of RA patients. In collagen-induced arthritis (CIA) and adjuvant-induced arthritis (AIA) models, treatment with a neutralizing antibody against cANGPTL4 (anti-cANGPTL4 Ab) markedly alleviated disease severity, suppressed inflammation, and prevented bone destruction. Transcriptomic and proteomic analyses of AIA synovial tissue showed that anti-cANGPTL4 Ab treatment inhibited fibroblast-like synoviocyte (FLS) migration and inflammation-induced osteoclastogenesis. Further mechanistic studies revealed that the anti-cANGPTL4 antibody suppresses TNF-α-induced inflammatory cascades in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS) via the Sirtuin 1/NF-κB signaling pathway. In addition, the study found that anti-cANGPTL4 antibody blocks FLS invasion- and migration-induced osteoclast activation. In summary, ANGPTL4 is a potential biomarker for RA diagnosis and treatment, and targeting cANGPTL4 offers a novel therapeutic strategy.
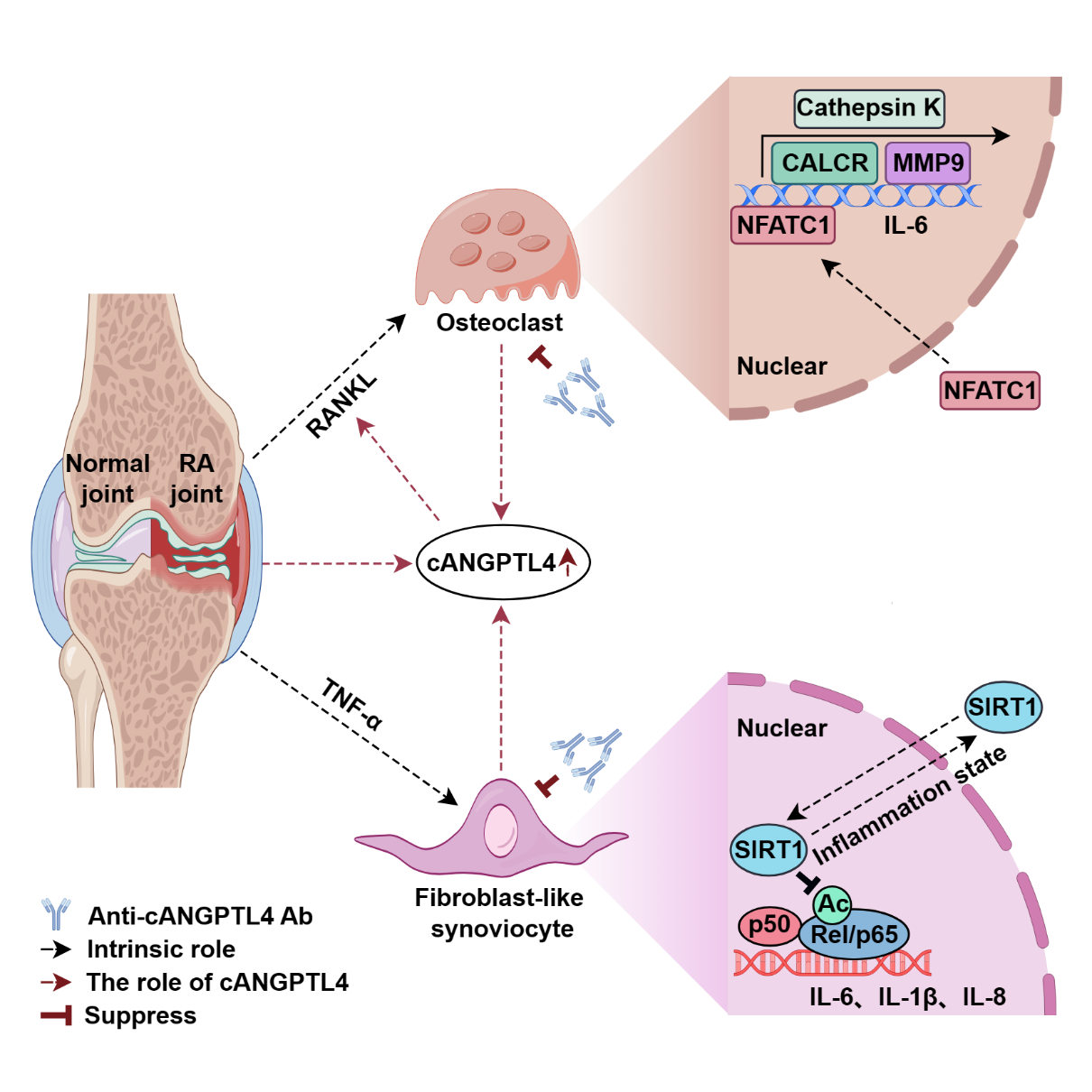
Study schematic
Ke Liqing (research assistant, Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences) and Dr. He Qifei (Shenzhen Second People’s Hospital) are co-first authors. Professor Zhang Peng (Shenzhen University of Advanced Technology), Assoc. Professor Cheng Wenxiang (Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences), and Professor Li Liang (Southern University of Science and Technology) are co-corresponding authors. We sincerely thank Professor Guo Jianping (Department of Rheumatology and Immunology, Peking University People’s Hospital), Professor Xu Jiake (Shenzhen University of Advanced Technology), and Professor Gong Xun (Guang’anmen Hospital, China Academy of Chinese Medical Sciences) for their valuable suggestions and constructive comments during the study and manuscript revision. This work was supported by the Key Program of the National Natural Science Foundation of China, the National Key R&D Program of the Ministry of Science and Technology, the Key Project of Guangdong Province, and the General Research Program of Shenzhen Healthcare Research Institute.