Alzheimer’s disease (AD), the most common form of dementia, is considered one of the most challenging major diseases facing humanity due to its insidious onset, prolonged course, and progressive worsening.
As indispensable research tools,AD animal modelsare widely used to investigate molecular mechanisms of AD pathogenesis, conduct preclinical drug testing, and identify reliable diagnostic biomarkers.
Recently,the team led by Professor Ye Keqiang from the Faculty of Life and Health Sciences at Shenzhen University of Advanced Technology published a review in Trends in Molecular Medicine (IF = 12.8). The article systematically reviews the most commonly used mouse models in AD research, highlights the latest advances in model construction, discusses their strengths and limitations, and provides an outlook on future directions in AD research.

Progress in the development of Alzheimer’s disease mouse models
Professor Ye Keqiang (Faculty of Life and Health Sciences, Shenzhen University of Advanced Technology) is thecorresponding author, and Associate Researcher Qian Zhengjiang (Shenzhen Institutes of Advanced Technology (SIAT), Chinese Academy of Sciences) is thefirst author.
The mainclinical symptoms of AD include cognitive and memory impairment, decline in daily living abilities, and behavioral/psychiatric abnormalities. Its hallmark pathological features are neurodegenerative changes in the central nervous system: massive neuronal loss, accompanied by extracellular senile plaques formed by β-amyloid (Aβ) peptide deposits and intracellular neurofibrillary tangles composed of hyperphosphorylated and truncated Tau protein.
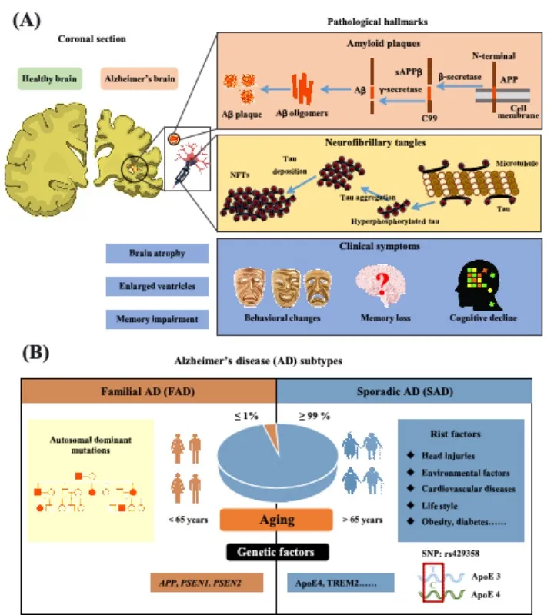
Pathological features and subtypes of Alzheimer’s disease
AD is classified into sporadic and familial forms. Sporadic AD accounts for >99% of cases and is driven by multiple factors, with ApoE4 being the strongest genetic risk factor. Familial AD (<1%) is primarily caused by mutations in genes encoding amyloid precursor protein (APP), presenilin-1, and presenilin-2 (PS1/PS2).
For decades, AD models have been predominantly built in rodents, with over 200 mouse models developed. The most common are transgenic mice overexpressing AD-related mutations in APP and PS1 (e.g., PDAPP, APP/PS1) to mimic Aβ pathology, and those overexpressing mutant Tau (e.g., JNPL3, PS19) to model Tau pathology—despite no Tau mutations being found in human AD patients. These transgenic models effectively recapitulate key AD pathological features but deviate significantly from human pathology due to artificial overexpression.
To better mimic physiological mutation-driven pathology, recent years have seen the rise of knock-in models such as APP NL-F and APP NL-G-F, as well as hybrids of these with other AD models. Models incorporating sporadic AD risk factors like ApoE4 and TREM2 (e.g., neuron-specific ApoE4 overexpression or TREM2 R47H knock-in) have also gained attention.
These models are crucial for deepening our understanding of sporadic AD pathogenesis.
Overall, while AD mouse models successfully replicate key pathological features, they fail to fully recapitulate the human disease progression. Significant evolutionary, genetic, and environmental differences between mice and humans pose major challenges for translational research based on these models.
As human lifespan increases, the rising number of AD patients will become a major societal burden. Future AD research will therefore focus onearly diagnosis, preventive treatment, and drug development.
Emerging approaches using PET imaging and fluid (especially blood-based) biomarkers for early diagnosis are rapidly advancing. Beyond mouse models, integrated multi-scale studies—including iPSC-derived cells, organoids, diverse animal models (including non-human primates), and clinical patient samples—will ultimately pave the way for effective AD therapies.
Thisstudy was supported by the Key Program of the National Natural Science Foundation of China (32330040), the Youth Program of NSFC (32200928), the Guangdong Basic and Applied Basic Research Foundation (2023A1515030296), the Shenzhen Science and Technology Innovation Program (JCYJ20220531100802005) and others.
.